Polyamines
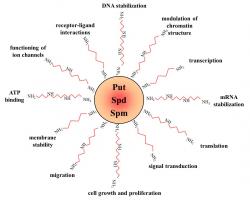
Polyamines are low molecular weight aliphatic polycations, highly charged and ubiquitously present in all living cells. Interest has been increasing during the last 30 years in the naturally abundant polyamines putrescine (diamine), spermidine (triamine) and spermine (tetramine), which were demonstrated to be involved in a large number of cellular processes. For example, polyamines participate in modulation of chromatin structure, gene transcription and translation, DNA stabilization, signal transduction, cell growth and proliferation, migration, membrane stability, functioning of ion channels and receptor-ligand interactions Polyamines seem to exert their role through ionic interactions, owing to their unique structural feature of regularly spaced positive charges.
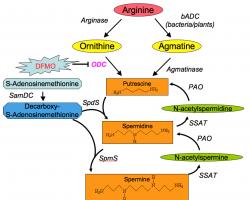
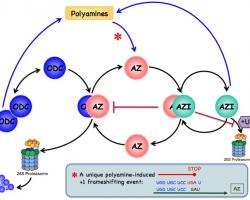
Inside the cell polyamines are present in nearly millimolar concentrations. There is equilibrium between polyamines that are bound to different polyanionic molecules (mainly DNA and RNA) and free polyamines. The free polyamine pool represents 7-10% of the total cellular polyamine content. Only the free intracellular polyamines are available for immediate cellular needsand therefore are subject to strict regulation. Polyamines are maintained within very narrow range because decrease in their concentrations inhibits cell proliferation while excess appears to be toxic. Therefore, the free polyamine pools are regulated in a very fast, sensitive and precise manner. This regulation is achieved at four levels: de novo synthesis, interconversion, terminal degradation and transport.