3D structures of cellulosomal modules
Crystal structures of cellulosomal modules
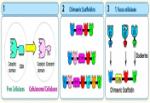
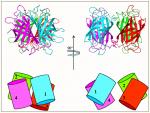
In order to understand the nature of the intermodular contacts formed between the cellulosomal subunits, or how polycellulosome assembly occurs, we employed crystallographic techniques. In early collaborative studies with Linda Shimon and Felix Frolow, we succeeded in determining the 3D crystal structure of a type-I cohesin. More recently, we successfully cloned, expressed, purified, crystallized and solved several novel structures of type-II and type-III cohesin modules from different cellulosomal species. A gallery of selected crystals grown in our lab is shown in Figure 1.