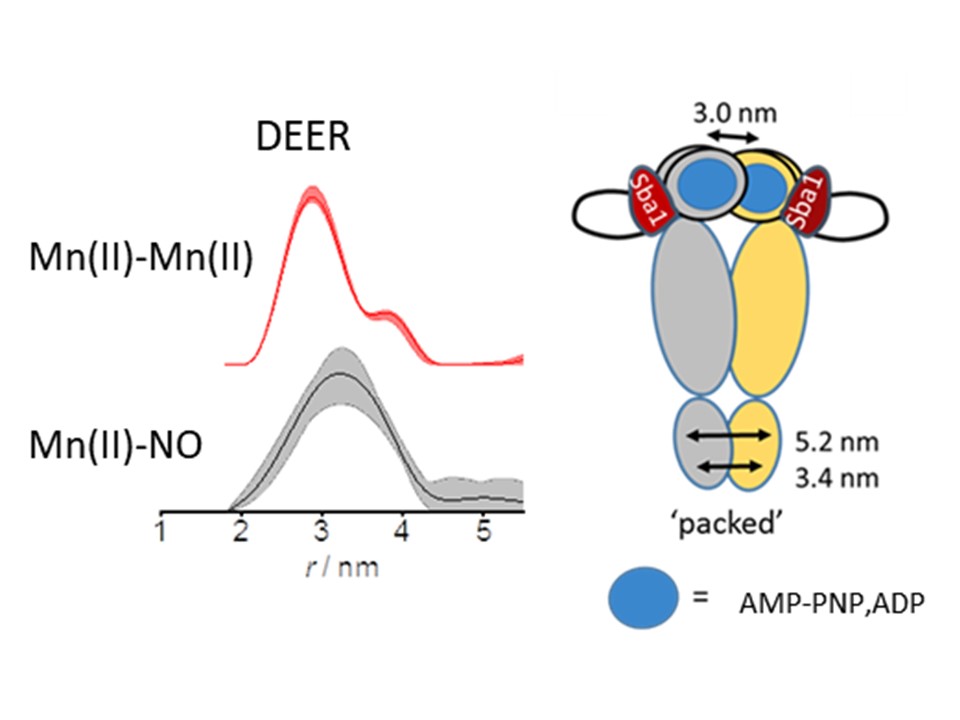
Hsp90 is an essential molecular chaperone of the cell that modulates the folding, activation, and stability depending on ATP hydrolysis of a plethora of client proteins represented in many types of cancer. Structurally, Hsp90 is a flexible homodimer with each protomer consisting of three highly conserved domains: The amino-terminal domain (NTD), where the ATPase site is located, the middle domain (MD) which is important for ATP hydrolysis and binding of clients, and the carboxyl-terminal domain (CTD) responsible for dimerization of the two monomers, that is essential for the function of the protein. The CTDs of Hsp90 are constitutively dimerized containing a binding site whereupon several co-chaperones bind at different stages of the ATPase cycle and modulate the conformation of Hsp90. I n the absence of nucleotide, the so called open apo-state, the NTDs are not dimerized and their relative positions are disordered, namely their inter-domain distances sample a large space. With nucleotide bound to the NTD, namely the bound state, the NTDs undergo an internal structure modification that leads to their association, forming a closed conformation.
The fact that many oncogenic proteins are clients of Hsp90, in combination with the high natural abundance of Hsp90 in the cell (1-2% of the cytosol, 10-150 μΜ), turned Hsp90 into a prime target for cancer treatment and has led to intensive investigation of Hsp90 inhibitors as cancer therapeutics. We are performing DEER distance measurements using non-conventional labeling schemes, including Gd(III), Mn(II) and nitroxides and their combinations, along with ENDOR experiments, to track the conformational changes of Hsp90 during the hydrolysis process in the presence of various co-chaperons and inhibitors. Initial studies are in vitro, to be followed by in-cell measurements.
References
-
Monitoring the Conformation of the Sba1/Hsp90 Complex in the Presence of Nucleotides with Mn(II)-Based Double Electron–Electron Resonance Giannoulis A., Feintuch A., Unger T., Amir S. & Goldfarb D. (2021) The journal of physical chemistry letters. 12, 51, p. 12235-12241. DOI: 10.1021/acs.jpclett.1c03641
-
Two closed ATP- and ADP-dependent conformations in yeast Hsp90 chaperone detected by Mn(II) EPR spectroscopic techniques Giannoulis A., Feintuch A., Barak Y., Mazal H., Albeck S., Unger T., Yang F., Su X. & Goldfarb D. (2020) Proceedings of the National Academy of Sciences of the United States of America. 117, 1, p. 395-404. DOI: 10.1073/pnas.1916030116


