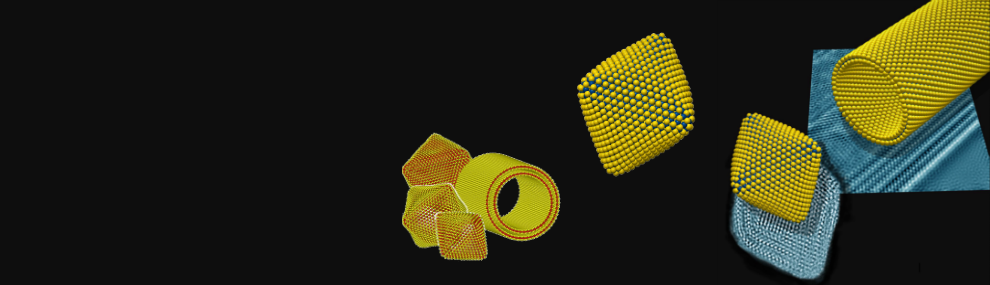
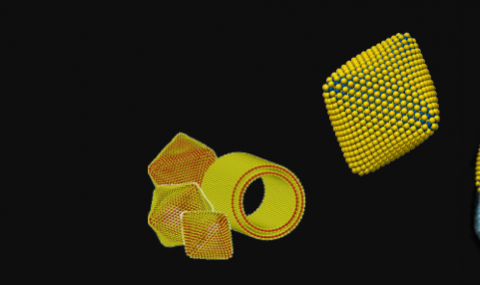
Inorganic Fullerene-like Nanostructures and Inorganic Nanotubes at the crossroad of solid-state chemistry and nanotechnology
In 1992 we showed that nanoparticles of the layered compound WS2 are unstable in the platelet form and they spontaneously form closed cage structures akin to carbon fullerenes and carbon nanotubes. This instability was attributed to the highly reactive dangling bonds of both sulfur and tungsten atoms, which appear at the periphery of the nanoparticles.
Numerous kinds of inorganic fullerene-like (IF) nanoparticles and nanotubes (INT) were synthesized by us and by others over the years. Furthermore, extensive in-silico studies by various groups were devoted to unravelling the properties of various inorganic nanotubes. Detailed study of the growth mechanism of fullerene-like WS2 nanoparticles and nanotubes was undertaken. This development led to the scaling-up of their production and their recent commercialization by NanoMaterials (www.apnano.com) and N.I.S. (www.nisusacorp.com) as superior solid lubricants with numerous potential applications. A production line for manufacturing lubricants (oils and greases) formulated with these nanoparticles as well as metal working fluids has been completed with sales exceeding 1000 metric tons of lubricants a year.
The mechanical, optical and electrical properties of WS2 nanotubes were carried out demonstrating the unique physical behavior of such 1D nanostructures. For example, jointly with Iwasa’s group in the University of Tokyo, these nanotubes were shown to exhibit (quasi-1D) superconductivity at about 5K and strong light emitting diodes were fabricated, as well.
Much of our efforts in the past and also today are focused on the synthesis of new nanotubes and fullerene-like nanoparticles and study of their unique physical and chemical behavior. Numerous nanotubes from the large family of “misfit” compounds, like PbS-NbS2, LaSe-TaSe2, have been recently prepared and studied. Doping of IF-MoS2 nanoparticles with minute amounts of Re and Nb atoms was demonstrated. Precipitous reduction in friction and wear was observed for lubricants formulated with the doped nanoparticles. Various medical applications were proposed for the doped IF nanoparticles.
The microscope image in the header above was produced by Drs. Maya Bar-Sadan and Lothar Houben of the Ernst-Ruska Centre for Microscopy and Spectroscopy with Electrons, in Jülich, Germany.
The models were calculated and designed by Prof. G. Seifert, TU Dresden and Dr. A.N. Enyashin, Institute of Solid State Chemistry, RAS, Ekaterinburg.
History of electron microscopy for materials at the Weizmann Institute
הרצאה (בעברית) במסגרת סדרת הרצאות "בכור המהפכה המדעית".
סדרת הרצאות שנתיות לזכרו של פרופ' אהרון קציר.
מנחה ההרצאה: פרופ' אברהם קציר מאוניברסיטת תל-אביב
Webinar hosted by the CEO of “Nanotech Industrial Solutions” (NIS), Mr. George Diloyan on August 29th 2020.
ראיון של פרופ' רשף טנא- חבר האקדמיה הלאומית הישראלית למדעים
מראיין: פרופ' יעקב קליין, חבר האקדמיה
IMEC-14 meeting, Tel Aviv (December 2009)
Keynote talk in Nanotechnology-Materials and Composites for Frontier Applications (NANOCON 012), Pune, Oct 2012
Nanoday-2018, Bilkent University, Ankara (May 2018)